-
Notifications
You must be signed in to change notification settings - Fork 0
How it Works

The script uses the uncontinuous score from a bigWig file to estimate footprints within the peaks of interest. A sliding window algorithm is used to look through the peaks. A region with significant high signal will be saved as a footprint. To estimate the significance of a footprint the signal is compared with the mean of the signals within the window. After the run the footprints are saved in a .bed file.
While using this script on it's own, the user can set the name for the output .bed file using the parameter --output_file. By default the output file is called footprints_extraction_output.bed and is saved to the working directory. While using the whole pipeline, the default name will be used for the output file to save it as an intermediate result.
For each footprint an individual score is found. The score shows the quality of the footprint and is found as the mean of the signals from bigWig file for the corresponding positions. The output .bed file has 8 columns and a header marked with #. The header identifies the names for all columns:
#chr start end name score len max_pos bonus_info
- chr for the chromosom;
- start for the start position of the footprint;
- end for the end position of the footprint;
- name for the unique name of the footprint;
- score for the quality of the footprint;
- strand for the strand of the footprint;
- len for the length of the footprint;
- max_pos for the relative position within the footprint, where the highest signal from the bigWig file is (if there are several positions with the highest score the position in the middle of those will be set as the max_pos);
- bonus_info for the additional information from the input .bed file.
If some information could not be defined, the point . will be written in the corresponding row and column. For example, if there was no bonus_info provided from the input .bed file, the point . could be found at the output file of the footprints_extraction.
During the run, a log-file is written. The log-file is called footprints_extraction.log and is saved to the working directory as well.
While using the script on it's own if the user does not want to receive the messages about the run in the terminal, the parameter --silent can be used to force the script write the messages only to the log-file.
Changing the optional parameters can lead to varying the number of found footprints and their length. For example, in the following picture shows three files. The first one is the .bigwig file with signal. The second is the output file produced with while setting the --percentage to 10, and the third is the output file produced with --percentage 0.
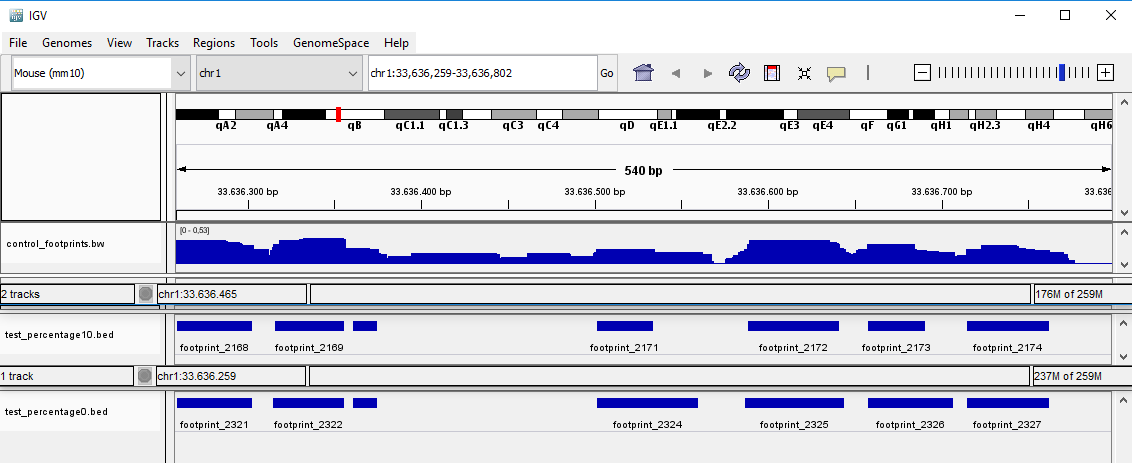
The smaller the --percentage is set, the longer the found footprints will be, but the quality of each footprint will be lower. The length of a window has also an impact on the length of found footprints. The bigger the --window_len parameter is set, the longer the found footprints will be, though this change is not as remarkable as while changing the --percentage parameter.
There is a possibility for a user to set the max allowed number of base pairs in between two footprints with the help of a parameter --min_gap. By default 6 bp are allowed. That means, all footprints, that have a smaller number of footprints in between, will be merged. The score for a merged footprint is calculated as a mean of scores of both original footprints. If the user doesn't want to merge the footprints, the --min_gap should be set to 1.
Footprints detected in the peak calling step 1.1 can belong to already annotated or to new, unknown motifs. This step (1.2) removes sequences in the footprints that are present in a set of known motifs. The calculation of the filtering is done with the bedtools command subtract. Non overlapping sequences pass the filter, if they are longer than a minimum length and shorter than the maximum length. The known motifs can either be calculated within this pipeline or the path to the directory where the known motifs bed-files are stored can be submitted. ##Parameters paths of parameters must not contain the "|" pipe-symbol.
The 3 required input parameters are:
- --fasta: Path to the fasta genome
- --data: Path to the bed file from output of 1.1 (9 or more column bed-file required)
- --motifs: Path to the directory of known motifs bed-file or comma separated list of files
- --min: min size of sequences (default is 10). Don't set this to 0 since very small sequences can be produced with bedtools subtract
- --max: max size of sequences (default is 200). Removes sequences greater than max. Can be set even higher since the clustering in 2.1 uses a k-mer approach which can basicly handle any length of sequences.
- --workdir: workdir is by default the current directory.
- --output: output path/file is by default at ./NewMotifs.bed and ./NewMotifs.fasta. If the output file name does not end with '.bed', the ending will be added.
The output bed-file contains the same information as the output file from 1.1 but with 2 added columns:
- a flag with possible values 1 or 0 which say if the point of maximum score of the original footprint lies inside or outside (1/0) after filtering.
- the DNA sequence

Example: The first footprint of the input is too short, it will not be present in the output. Non overlapping regions of the other two footprints with known motifs that are longer than min length are added to the output BED-file. Splitted footprints get a new id appended, starting with .0
An additional output, compareBed.stats, with information about the run is created. It contains the columns:
- sum_nt_input -> the total count of nucleotides of all footprints in the input data
- sum_nt_filtered -> the total count of nucleotides of all footprints in the output data
- quotient_of_nt -> sum_nt_input / sum_nt_output
- loss_of_nt -> 1 - sum_nt_output/sum_nt_input
- mean_length_input
- mean_length_filtered
- flag_1_ratio -> ratio of ((entries with flag == 1) / all entries)
- initial number of footprints
- number of footprints after subtract
The remaining footprints from the previous step are now clustered for later motif generation. This process is divided into sequence reduction and the actual clustering. For reduction each of the given sequences (= footprints) is shortened to a conserved region resulting in an overall reduced background noise in the data. This is necessary since motifs, due to their size, cover only a small portion of the footprint. Thereafter CD-HIT is utilized for clustering.
Reducing given sequences to dense regions is crucial to produce clean clusters subsequently. This is done with a k-mer approach through the following steps: 1. Count k-mer 2. Compute k-mer cutoff and remove k-mer below this cutoff 3. Find positions of remaining k-mer on each sequence 4. Merge overlapping k-mer 5. Reduce each sequence to it's best region
The cutoff is calculated by assuming that 100% (parameter: -f) of the sequences contain a motif of set length (parameter: -m). Now with the length of each sequence it can be computed how many k-mer positions are possible that overlap at least half of the motif (parameter: -v). This results in the number of interesting k-mer over the whole dataset.

Then the k-mer are ordered descending after their count and the cumulative sum is calculated. All k-mer with a cumulative sum below the threshold are kept and the ones above discarded.

After the k-mer are reduced to significant ones they are compared back to the original sequences. For each sequence k-mer that overlap with at least k-mer - 1 nucleotides (parameter: -n) are merged to a single component. Those components consisting of one or more k-mer are then compared and the component with the highest score is used as the reduced sequence. The score of each component is the sum of counts of all their k-mer. If a sequence contains no k-mer it will be removed.

Basic command:
Rscript reduce_sequence.R -i input.bed
Parameter:
Usage: reduce_sequence.R [options]
Reduces each sequence to its most frequent region.
Options:
-i CHARACTER, --input=CHARACTER
Input bed-file. Last column must be sequences.
-k INTEGER, --kmer=INTEGER
K-mer length. Default = 10
-m INTEGER, --motif=INTEGER
Estimated motif length. Default = 10
-o CHARACTER, --output=CHARACTER
Output file. Default = reduced.bed
-t INTEGER, --threads=INTEGER
Number of threads to use. Use 0 for all available cores. Default = 1
-c LOGICAL, --clean=LOGICAL
Delete all temporary files. Default = TRUE
-s INTEGER, --min_seq_length=INTEGER
Remove sequences below this length. Defaults to the maximum value of motif and k-mer and can not be lower.
-n INTEGER, --minoverlap_kmer=INTEGER
Minimum required overlap between k-mer. Used to create reduced sequence ranges out of merged k-mer. Can not be greater than k-mer length. Default = kmer - 1
-v INTEGER, --minoverlap_motif=INTEGER
Minimum required overlap between motif and k-mer to consider k-mer significant. Used for k-mer cutoff calculation. Can not be greater than motif and k-mer length. Default = ceiling(motif / 2)
-f DOUBLE, --motif_occurrence=DOUBLE
Define how many motifs are expected per sequence. This value is used during k-mer cutoff calculation. Default = 1 meaning that there should be approximately one motif per sequence.
--summary=CHARACTER
Filepath where a small summary file of the run should be written. Default = NULL
-h, --help
Show this help message and exit
Author: Hendrik Schultheis <Hendrik.Schultheis@mpi-bn.mpg.de>
The clustering script takes a bed-file as input and appends a column with cluster numbers. It is mostly a wrapper function for CD-HIT-EST with new default parameter for this specific pipeline.
Basic command:
Rscript cdhit_wrapper.R -i input.bed
Parameter:
Usage: cdhit_wrapper.R [options]
CD-HIT-EST Wrapper function. See https://github.com/weizhongli/cdhit/wiki for more information regarding CD-HITs parameters.
Options:
-i CHARACTER, --input=CHARACTER
Input bed-file. Fourth column is expected to contain names, last column must be sequences.
-c DOUBLE >= 0.8, --identity=DOUBLE >= 0.8
Identity threshold. Default = 0.8 (CD-HIT parameter)
-A INTEGER, --coverage=INTEGER
Minimal alignment length for both sequences in nucleotides. Default = 8 (CD-HIT parameter)
-o CHARACTER, --output=CHARACTER
Output file same as input but with appended column of cluster numbers. Default = cluster.bed
--clean=LOGICAL
Delete all temporary files. Default = TRUE
-G INTEGER, --global=INTEGER
Global sequence identity = 1, local = 0. Default = 0 (CD-HIT parameter)
-b INTEGER, --band_width=INTEGER
Alignment band width. Default = 20 (CD-HIT parameter)
-M INTEGER, --memory=INTEGER
Memory limit in MB. 0 for unlimited. Default = 800 (CD-HIT parameter)
-T INTEGER, --threads=INTEGER
Number of threads. Default = 1 (CD-HIT parameter)
-n INTEGER, --word_length=INTEGER
Default = 3 (CD-HIT parameter)
-l INTEGER, --throw_away_sequences=INTEGER
Maximum length of sequences thrown away. Default = 5 (CD-HIT parameter)
-s DOUBLE, --length_dif_cutoff_shorter_p=DOUBLE
Shorter sequences length must be at least x percent of longer sequences length. Default = 0 (CD-HIT parameter)
-S INTEGER, --length_dif_cutoff_shorter_n=INTEGER
Length difference between sequences can not be higher than x nucleotides. Default = 999999 (CD-HIT parameter)
-e DOUBLE, --alignment_coverage_longer_p=DOUBLE
Alignment must cover x percent of longer sequence. Default = 0 (CD-HIT parameter: -aL)
-E INTEGER, --alignment_coverage_longer_n=INTEGER
There can not be more than x unaligned nucleotides on the longer sequence. Default = 99999999 (CD-HIT parameter: -AL)
-f DOUBLE, --alignment_coverage_shorter_p=DOUBLE
Alignment must cover x percent of shorter sequence. Default = 0 (CD-HIT parameter: -aS)
-F INTEGER, --alignment_coverage_shorter_n=INTEGER
There can not be more than x unaligned nucleotides on the longer sequence. Default = 99999999 (CD-HIT parameter: -AS)
-w DOUBLE, --max_unmatched_longer_p=DOUBLE
Maximum unmatched percentage on longer sequence (excludes leading tailing gap). Default = 1 (CD-HIT parameter: -uL)
-W DOUBLE, --max_unmatched_shorter_p=DOUBLE
Maximum unmatched percentage on shorter sequence (excludes leading tailing gap). Default = 1 (CD-HIT parameter: -uS)
-U INTEGER, --max_unmatched_both_n=INTEGER
Maximum unmatched nucleotide on both sequences (excludes leading tailing gap). Default = 99999999 (CD-HIT parameter)
-g INTEGER, --fast_cluster=INTEGER
Cluster sequence in first cluster that meets threshold = 0 (fast). Or cluster in best Cluster = 1 (accurate). Default = 1 (CD-HIT parameter)
-r INTEGER, --strand=INTEGER
Align +/+ & +/- (= 1). Or align only +/+ (= 0). Default = 0 (CD-HIT parameter)
--match=INTEGER
Matching score. Default = 2 (CD-HIT parameter)
--mismatch=INTEGER
Mismatch score. Default = -2 (CD-HIT parameter)
--gap=INTEGER
Gap score. Default = -6 (CD-HIT parameter)
--gap_ext=INTEGER
Gap extension score. Default = -1 (CD-HIT parameter)
-x INTEGER, --sort_cluster_by_size=INTEGER
Either sort cluster by decreasing length (= 0) or by decreasing size (= 1). Default = 1 (CD-HIT parameter: -sc)
--summary=CHARACTER
Filepath where a small summary file of the run should be written. Default = NULL
-h, --help
Show this help message and exit
Author: Hendrik Schultheis <Hendrik.Schultheis@mpi-bn.mpg.de>
The process BED_to_FASTA calls the R-script bed_to_fasta.R, which extracts the sequences from a given BED-file and creates a FASTA-file per cluster. The cluster is given by an additional column in the BED-file.
Usage: bed_to_fasta.R [options]
Convert BED-file to one FASTA-file per cluster.
Options:
-i CHARACTER, --input=CHARACTER
Input bed-file. Second last column must be sequences and last column must be the cluster id.
-p CHARACTER, --prefix=CHARACTER
Prefix for file names. Default = ''
-m INTEGER, --min_seq=INTEGER
Minimum amount of sequences in clusters. Default = 100
-h, --help
Show this help message and exit
The process GLAM2 uses the FASTA-files created from BED_to_FASTA as input and calls GLAM2. GLAM2 is a tool provided inside the MEME Suite. "GLAM2 is a program for finding motifs in sequences, typically amino-acid or nucleotide sequences. The main innovation of GLAM2 is that it allows insertions and deletions in motifs." GLAM2 uses Dirichlet processes to align the sequences and find motifs inside the sequences. GLAM2, by default, generates ten motifs per cluster. The output is a MEME-file for each cluster, which contains all ten motifs.
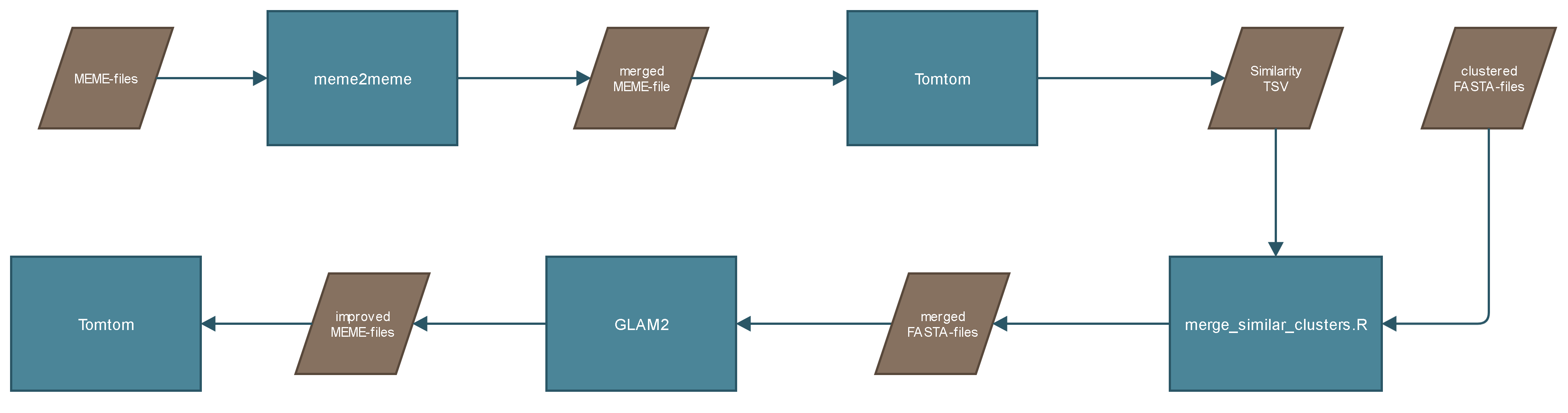
Because the previous done clustering step does not create perfect clusters. The motifs created from GLAM2 are clustered. To cluster the motifs all MEME-files are merged with meme2meme, a tool provided inside the MEME Suite. The merged MEME-file is compared with itself by Tomtom. Tomtom is a tool provided inside the MEME Suite and "compares one or more motifs against a database of motifs (e.g., JASPAR). Tomtom will rank the motifs in the database and produce an alignment for each significant match". The similarity between the motifs gives the information about the cluster similarity. The output would look something like this:

The first two columns are the important ones. Both columns contain two numbers separated by a dot (e.g. 1.1 or 2.10). The first number stands for the motif number. So for each cluster 10 motifs are created. The second number stands for the cluster number. So '2.10' means that its the second motif from the tenth cluster. Looking at the fifth row the information you get from it is that motif 1 from cluster 1 is similar to motif 5 from cluster 3, which supposes that cluster 1 is similar to cluster 3.
The cluster numbers are extracted from the file, which creates a table with two columns. This table can be interpreted as an edge-list for creating a weighted graph. Before creating the graph identical column values are removed:
Ideally, the graph forms clusters:

Depending on those improved clusters the FASTA-files from corresponding clusters are merged. The merged FASTA-files are given to GLAM2 to find the further improved motifs.
The process Tomtom gets the MEME-files from the Motif clustering and a database with known motifs as input. It compares the Motifs to a Motif-database with Tomtom.
If tomtom can find the motif in the database the cluster is sorted out. This is done with groovy inside the nextflow script. No separate script is called for this action.
GLAM2 does not format its output files in the right MEME-format. The script get_best_motif.py converts the files to the correct MEME-format. It also saves only the best X (Default: 3) Motifs.
usage: get_best_motif.py [-h] meme output num id
A script to convert from GLAM2 output to MEME-format and parsing only the
[num] first motifs from file to the output.
positional arguments:
meme Path to 'meme' file generated by GLAM2
output Output file
num Number of motifs parsed from file
id Cluster ID
optional arguments:
-h, --help show this help message and exit
Parallel to get_best_motif the script get_motif_seq.R is called. This script creates a JSON-file which contains the sequence names, which were used by GLAM2 to create the motifs.
Usage: get_motif_seq.R [options]
Creating JSON-file with sequence ids which were used to create the best (num) motifs.
Options:
-i CHARACTER, --input=CHARACTER
Input file. Output txt-file from GLAM2.
-o CHARACTER, --output=CHARACTER
Output JSON-file. Default = 'sequences.json'
-n NUMERIC, --num=NUMERIC
Get best (num) motifs. Default = '3'
-c NUMERIC, --cluster_id=NUMERIC
Cluster ID
-t CHARACTER, --tmp=CHARACTER
Path for tmp-files. Default = './'
-h, --help
Show this help message and exit
The GTF-Creation-Tool (RegGTFExtractor) creates GTF-Files with regulatory features based on the source organism. For this, it utilizes the Ensembl Regulatory Build and the RefSeq-FuncElem-Track from UCSC Table-Browser to gather data for regulatory features. The extracted data is saved in a GTF-formatted file. Additionally, activity data for different tissue types (based on JSON config) is gathered and added to the attributes field from the output file. Supported organism assemblies are: human: hg19, hg38, mouse: mm9, mm10. The regulation data is always extracted for the current assembly version (hg38 and mm10), if an older version is chosen (hg19 or mm9) the resulting GTF-output is automatically converted via CrossMap.
Basic command:
./RegGTFExtractor.py organism
Required parameter:
organism STRING the source organism (possible inputs: "hg19"/"hg38"/"mm9"/"mm10")
Optional parameter:
--tissues STRING Filter for tissue/category activity categories as in JSON config (Default: None)
--wd PATH current working directory for the script (default: ".")
--dir PATH directory for saved Ensembl / UCSC file (default: "./data/")
--out PATH Path to output-file (default: "./[organism].gtf")
This tool has the following dependencies:
- Python 3 or newer
- Linux 64-bit
- CrossMap
The Results have a customizable activity-list in their attributes field. This list is changed via config. The config is in JSON-Format, and contains a list of category-elements with three fields:
- type = Name of the category
- alias_ucsc = Aliasname in UCSC datafiles (Name of the folder in activity subfolder)
- alias_ensembl = Aliasname in Ensembl datafiles (Alias from UCSC Bed-file)
Example:

The activity can have the following status, based on Ensembl scheme:
- ACTIVE
- POISED
- REPRESSED
- INACTIVE
- NA
If multiple aliases are assigned to the same category, the activity will get the "highest" activity status. The order for activity is listed above. Only the highest activity will count. e.g. if in a feature one alias is ACTIVE and the other INACTIVE, the result will be noted as ACTIVE.
The resulting File is in GTF-Format. In which each line contains one regulatory feature. It has the following columns, with no header:
[chromosome] [source] [feature] [start] [end] [score] [strand] [frame] [list of attributes]
Example Results:

In the attributes field are the two values: gene_id (Ensemble: ENSR-ID or UCSC: RefSeq-ID) and activity: a list of categories from config with their activity-status for this feature. If cross-mapping is enabled and an older assembly version is chosen, in addition to the resulting GTF-File a ".unmap" file is created, this file contains entries from the original file that could not be found in the older assembly version.
The re-scan step uses tfbsscan to look for the new found motifs in a given genome. A BED-file for each cluster is created, which contains all found sites.
This process calls the venn.R script. It compares the sequences which created the motif for a given cluster with by re-scan found locations on the genome. It creates a Venn diagram of both sequences.
/images/venn_example.png Blue: sequences which created the motif. Red: New locations found with re_scan.